What is ocean acidification?
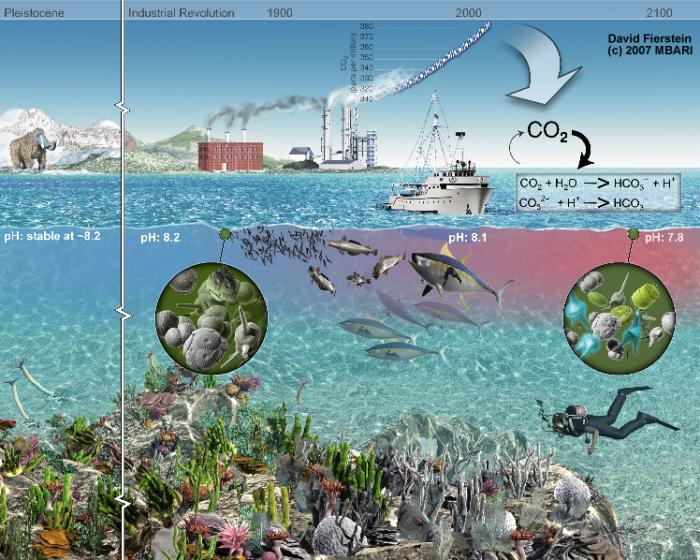
During the last 200 years water has become 30% more acidic, faster than any change recorded in the last 50 million years. The reason is that due to industrialisation there appear higher concentrations of CO2 in the atmosphere which dissolve in the ocean and makes it more acidic.
Ocean acidification is characterised by a low pH value, high CO2 concentrations and high amounts of H+ (hydrogen ions).
What is it caused by and what are some of its consequences?
30% of CO2 in the air dissolves into the sea where it mixes with water and carbonic acid is created:
CO2+H2O= H2CO3
H2CO3 releases H+. The more the ocean contains, the more acidic it is. Therefore there is a correlation between CO2 level in atmosphere, seawater and acidification also as H+ and pH. Lower pH values describe higher acidification.
This drop in pH has drastic consequences for organisms. Corals and shells dissolute in an acidic environment and have increased energy demands to build and maintain their shells. In general there is less marine biodiversity. Organisms are not able to cope with rapid changes in pH in the ocean. They have evolved over millions of years with a stable pH in the ocean where CO2 levels were lower as today. Thus they are not able to respond to fast changes that happen now. As a consequence organisms have a reduced metabolism, reduced oxygen uptake and reproductive success, changes in respiration and stress. Chemical changes are predictable, biological adaptions are not. Some species will flourish under these new circumstances while others will extinct. It is expected that ocean acidification will have impacts on fisheries and aquaculture, food security, tourism and sea-related economies.
References
The Ocean Portal Team (2018) Ocean acidification, Smithsonian Ocean [online] Available from: https://ocean.si.edu/ocean-life/invertebrates/ocean-acidification
Boundary, Rob (2010) The threat of ocean acidification, TED [online] Available from:
Illustration
Drs. Bralower, Timothy and Bice, David (n.d.) Ocean acidification, College of Earth and Mineral Science, The Pennsylvania State University [online] Available from: https://www.e-education.psu.edu/earth103/node/647


Leave a comment